Changes in nuclear pore numbers control nuclear import and stress response of mouse hearts
ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px; font-family: NexusSerif, Georgia, "Times New Roman", Times, STIXGeneral, "Cambria Math", "Lucida Sans Unicode", "Microsoft Sans Serif", "Segoe UI Symbol", "Arial Unicode MS", serif; font-size: 18px;">Nuclear pores are so essential for nuclear-cytoplasmic transport. Whether and also how cells change nuclear pores to alter nuclear transport and cellular function is unknown. Here, we try to show that rat heart muscle cells which mean (cardiomyocytes) undergo a 63% is decrease in nuclear pore numbers during maturation, and this changes their responses to extracellular signals.
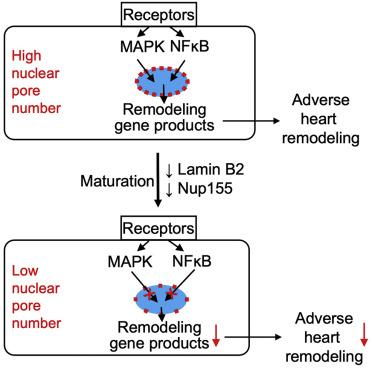
The maturation-associated decline in nuclear pore numbers is associated with the lower nuclear import of signaling proteins such as mitogen-activated protein kinase also known as MAPK. According to the experimental reduction of nuclear pore numbers decreased nuclear import of signaling proteins, resulting in decreased expression of immediate-early genes. In a mouse model of high blood pressure, the reduction of nuclear pore numbers improved adverse heart remodeling and reduced progression to lethal heart failure. The decrease in the nuclear pore numbers in cardiomyocyte maturation and resulting functional changes demonstrate how terminally it is differentiated cells permanently alter their handling of information flux across the ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px;">nuclear envelope and, with that, their behavior.
The ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px;">nuclear envelope is a double ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px;">phospholipid bilayer that used to separate the nucleus from the cytoplasm. ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px;">Nuclear pores ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px;">transport macromolecules into and out of the nucleus at a rate of several hundred molecules per second, and this includes ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px;">signal transduction proteins that regulate ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px;">gene transcription. Whether and how cells alter NPs to modulate the access of signal transduction proteins into nuclei and, with that, their transcriptional and functional responses, is unknown.
One property that can be assessed is the number of NPS per nucleus. NP numbers are widely between cell types and species: whereas yeasts have approximately 100 NP per nucleus, newt heart cells have approximately 12,000 Maul and Deaven, 1977. NP numbers are constant in the adult rat liver and brain cells, and this suggests that cells establish the NP numbers for their life when they terminally differentiate between D'Angelo et al.,2009. We present the observation that heart muscle cells which mean (cardiomyocytes) decrease the number of their NPS during maturation toward the post-mitotic state, and we have provided evidence for the significance of this decrease for nuclear transport and the response of the heart to stress.
After mammalian cardiomyocytes enter the terminally differentiated state and become post-mitotic, they grow by enlargement (hypertrophy). In mice and rats, this transition occurs within weeks after birth and is indicated by the formation of binucleated cardiomyocytes (Kirillova et al., 2021). Although hypertrophy is a mechanism of developmental heart growth, in terminally differentiated cardiomyocytes, cardiac stress induces maladaptive hypertrophy, and this leads to pathological remodeling of the heart, ultimately resulting in heart failure. Cardiac remodeling due to high blood pressure is a significant public health problem Heineke and also Molkentin,2006. Cardiac maladaptive remodeling is initiated by extracellular factors that activate G-protein coupled receptors (GPCRs) and receptor tyrosine kinases (RTKs), which activate intracellular signaling pathways, chiefly the mitogen-activated protein kinase (MAPK) pathways, leading to gene transcription changes Heineke and also Molkentin,2006.
Although it is cardiac maladaptive signaling at the level of GPCR, RTK, MAPK, and gene transcription is well understood, the fundamental mechanisms which could alter the propagation of these signals into the nucleus to determine the maladaptive response in cardiomyocytes are unknown. We have also developed a hypothesis based on our observation that the number of NPs decreases during cardiomyocyte maturation. We also try to present testing of the hypothesis that the decrease of NP numbers decreases nuclear import of the signaling proteins, and for this changes, adaptive gene transcription, and, with that, improves the cardiac response to stress.
Conclusion:
- Cardiomyocytes decrease their NP numbers during maturation
- NPs are cylindrical macromolecular complexes of approximately 120–180 nm outer diameter. Multiples of ∼34 different proteins, called ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; text-decoration-line: underline; text-decoration-thickness: 1px; text-decoration-color: rgb(46, 46, 46); color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px;">nucleoporins (Nups), are assembled into NPs consisting of ∼1,000 Nups.
- Nups are arranged in 8-fold symmetry around a ∼5 nm central opening. Due to their very large size, individual NPS can be visualized with microscopy. By using immunofluorescence ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; text-decoration-line: underline; text-decoration-thickness: 1px; text-decoration-color: rgb(46, 46, 46); color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px;">confocal microscopy, we visualized NPs in less mature (fetal and neonatal) and terminally differentiated adult rat ventricular cardiomyocyte nuclei.
- Quantification showed us that the number of NPS per nucleus decreased from 1,856 ± 66 NPs in fetal (E14.5) to 1,040 ± 53 in neonatal (P2), and further to 678 ± 32 in adult (P60) rat cardiomyocytes. This overall 2.7-fold decrease corresponds to a 63% decline in the ScienceDirect's AI-generated Topic Pages" class="topic-link" style="margin: 0px; padding: 0px; text-decoration-line: underline; text-decoration-thickness: 1px; text-decoration-color: rgb(46, 46, 46); color: rgb(46, 46, 46); word-break: break-word; text-underline-offset: 1px;">NP number.
Story Source:
Materials provided by CELL - Developmental Cell- Current. The original text of this story is licensed under a Creative Commons License. Note: Content may be edited for style and length.
Journal Reference: Science direct
0 Comments