How Plants Count to Six: Decoding Stomatal Responses Through Calcium Signaling
How Plants Count to Six: The Surprising Science Behind Stomatal Regulation
Plants have developed a fascinating way to manage water consumption: they use tiny adjustable pores called stomata, which open and close in response to environmental conditions. When there is plenty of water and light, these stomata open to allow the intake of carbon dioxide for photosynthesis. In contrast, during droughts or darkness, they close up to conserve water. But how exactly do plants make this decision? The secret lies in the intricate workings of guard cells and their calcium signaling mechanisms.
The Role of Guard Cells and Anion Channels
Stomata are controlled by pairs of guard cells that surround each pore. These cells play a crucial role in regulating stomatal movement based on external conditions. Central to this process are SLAC/SLAH-type anion channels located in the guard cells. Research led by Professor Rainer Hedrich at Julius-Maximilians-Universität (JMU) Würzburg in Germany has shown that these channels are essential for stomatal regulation, being directly activated by calcium signals.
Calcium signaling in guard cells is triggered by various environmental stimuli, such as drought, nutrient deficiencies, soil salinization, or pathogen attacks. These signals often manifest as "calcium transients"—brief, rapid increases in calcium concentration within the cells. Interestingly, the nature of these signals can differ depending on the specific environmental trigger, leading scientists to refer to them as "calcium signatures."
The All-or-Nothing Response of Calcium Transients
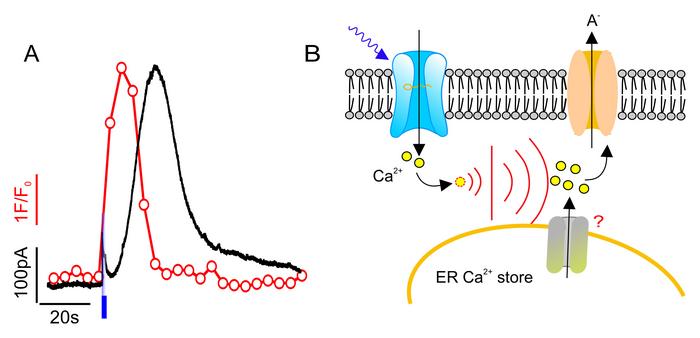
To uncover how much information is encoded in a calcium transient, Hedrich's team employed an innovative optogenetic approach using model plants with light-activated calcium channels. By exposing the guard cells to light pulses of varying durations (0.1, 1, and 10 seconds), they generated calcium signals to observe the cellular response.
The results were surprising: regardless of the pulse duration, the calcium transient in the guard cells remained remarkably similar. The calcium concentration would spike for about 30 seconds following the light stimulus and then gradually return to normal over the next 30 seconds. This "all-or-nothing" response suggested that once calcium enters the cells, it triggers a further release of calcium from internal stores, amplifying the signal.
The research confirmed this hypothesis. When the calcium storage within the cell's endoplasmic reticulum was blocked, the usual calcium transient and subsequent stomatal response did not occur, highlighting the importance of these internal calcium stores.
Delayed Anion Currents and Stomatal Closure
The team's findings didn’t stop there. They also noticed a delayed electrical response in the guard cells, known as the anion current, which followed the calcium signal. Just like the calcium transients, different pulse durations resulted in anion currents of similar strength and shape. However, the anion current only began after the calcium concentration had surpassed a specific threshold in the cytosol, and it persisted for about 30 seconds after the calcium levels subsided.
This time lag can be attributed to the biology of the enzymes that respond to the calcium signal and regulate the activity of the anion channels. Essentially, a 0.1-second calcium influx can be amplified within the cell to trigger a reaction lasting over a hundred times longer.
Counting Calcium Transients: How Guard Cells "Count to Six"
An even more intriguing discovery emerged when the researchers examined how many calcium transients were necessary for stomatal closure. They exposed the guard cells to a brief light pulse every 30 seconds, observing a progressive narrowing of the stomata. After the first pulse, the pore width decreased by 10%. With three pulses, it shrank by 30%, and after six, by 80%. After 12 or more pulses, the pores were completely closed.
This finding led to a surprising conclusion: guard cells can "count" up to six calcium transients, converting these signals into a corresponding stomatal movement. Notably, altering the frequency of stimulation had a clear impact. When the frequency was doubled, stomatal closure did not happen any faster, and when halved, the process slowed down.
What’s Next? Unraveling the Stomatal Mystery
The research opens up several new questions. "We’re currently investigating the steps in the stimulus-response chain that depend on the frequency of the calcium transient and affect the speed of stomatal response," says Professor Hedrich. The team is also exploring how guard cells decode the calcium signals and translate them into an enzyme-driven activation of anion channels based on the number of signals. Another key question is how long guard cells can "remember" each calcium transient.
This exploration of plant signaling pathways not only provides a deeper understanding of how plants manage water resources but may also offer insights for developing crops better adapted to changing environmental conditions.
Story Source:
Materials provided by University of Würzburg. The original text of this story is licensed under a Creative Commons License. Note: Content may be edited for style and length.
Journal Reference:
- Shouguang Huang, M. Rob G. Roelfsema, Matthew Gilliham, Alistair M. Hetherington, Rainer Hedrich. Guard cells count the number of unitary cytosolic Ca2+ signals to regulate stomatal dynamics. Current Biology, 2024; DOI: 10.1016/j.cub.2024.07.086

0 Comments