Rice University's Game-Changing Reactor for Efficient Carbon Capture
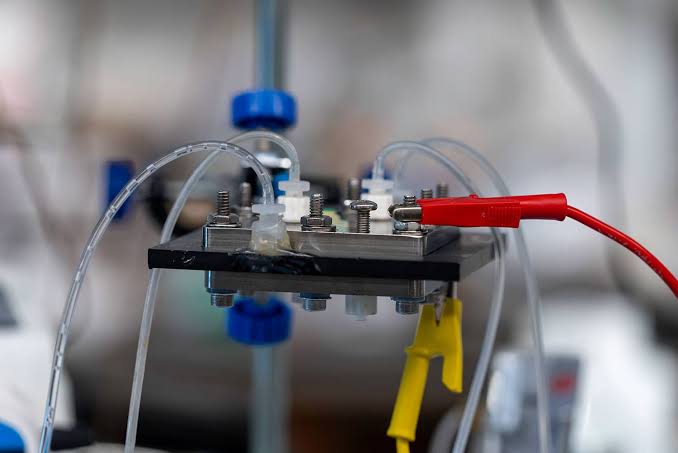
Rice University researchers have pioneered an electrochemical reactor that could drastically reduce energy consumption in capturing carbon dioxide directly from the atmosphere. This innovative device addresses the pressing need for scalable, cost-effective solutions to reduce atmospheric CO₂ emissions, with potential to become a cornerstone of future climate mitigation efforts.
In a study published in *Nature Energy*, the team describes a reactor design with a modular, three-chamber structure centered on a porous solid electrolyte layer. Haotian Wang, a Rice University chemical and biomolecular engineer leading the study, called it “a big milestone in carbon capture from the atmosphere,” emphasizing its relevance for industrial applications. “Our research findings present an opportunity to make carbon capture more cost-effective and practically viable across a range of industries,” Wang noted, underscoring the technology’s versatility and wide-scale potential.
How It Works: Innovative Reactor Design
The reactor has achieved impressive rates of carbon dioxide regeneration, demonstrating stability and adaptability with various cathode and anode reactions. Wang highlighted the technology's unique flexibility, explaining that it can operate with multiple chemistries and even coproduce hydrogen—an advantage that could significantly lower capital and operational costs for industries transitioning to net-zero fuels and chemicals.
Unlike conventional direct air capture methods, which require high temperatures to extract CO₂ from mixed gas streams, Rice’s electrochemical approach bypasses these energy-intensive steps. Traditional systems filter CO₂ by running a gas stream through high-pH liquids, creating bonds that require substantial heat, chemicals, or additional electrochemical processes to break. In contrast, this new reactor uses electrical energy instead of thermal energy, functioning at room temperature, eliminating the need for added chemicals, and generating no harmful byproducts.
The Challenge of CO₂-Trapping Chemicals
Capturing carbon dioxide from air typically requires sorbents that chemically bind with CO₂. Amine-based sorbents, popular for their energy-efficient bond breaking, are toxic and less stable. Water-based solutions, which are greener, require higher temperatures for CO₂ release. The Rice team’s design offers a better alternative, efficiently separating carbonates and bicarbonates into an alkaline absorbent and high-purity CO₂, using optimized electrical inputs to control ion movement and minimize energy costs.
Looking Ahead
Wang hopes this breakthrough will motivate more industries to adopt sustainable practices, fueling momentum toward a net-zero future. "Rice is the place to be if you are passionate about sustainability and energy innovation," he said. This project, along with others led by his lab, aligns with Rice University’s commitment to sustainability in energy research.
The study’s authors include former Rice postdoctoral researcher Xiao Zhang, Rice doctoral alumni Peng Zhu and Yang Xia, and co-first author Zhiwei Fang. The work was supported by the Robert A. Welch Foundation and the David and Lucile Packard Foundation.
Story Source:
Materials provided by Materials provided by Rice University. Original by Silvia Cernea Clark.. The original text of this story is licensed under a Creative Commons License. Note: Content may be edited for style and length.
Journal Reference:
- Xiao Zhang, Zhiwei Fang, Peng Zhu, Yang Xia, Haotian Wang. Electrochemical regeneration of high-purity CO2 from (bi)carbonates in a porous solid electrolyte reactor for efficient carbon capture. Nature Energy, 2024; DOI: 10.1038/s41560-024-01654-z

0 Comments