WashU Medicine Identifies New Genetic Disorder Linked to Brain Malformations
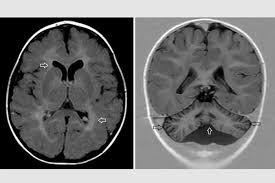
Researchers at Washington University School of Medicine, in collaboration with an international team of doctors and scientists, have identified the cause of a rare disorder that involves intellectual disability and brain malformations. The study, published on October 31 in *Science*, links the child's neurological symptoms to a genetic change affecting protein folding within cells, leading to a molecular diagnosis and the identification of a new type of genetic disorder.
For the 30 million Americans living with rare diseases, achieving a diagnosis can be a long and arduous journey. Many patients present symptoms that do not align with known disease patterns, resulting in diagnostic odysseys that can span years. This cross-disciplinary team has shed light on a case that previously defied categorization, providing crucial insights into the condition of a child from Germany exhibiting symptoms such as intellectual disability, low muscle tone, and abnormal brain structures.
The team, led by Stephen Pak, PhD, a professor of pediatrics at WashU Medicine, found a mutation in the CCT3 gene, which they hypothesized could be responsible for the child’s condition. They utilized *Caenorhabditis elegans* (C. elegans), a roundworm model organism, to investigate the effects of this genetic variant. Their findings revealed that the worms with the patient’s mutation exhibited slower movement compared to those with a healthy gene copy, indicating that the genetic change impacts mobility and nervous system function.
The CCT3 protein is part of the TRIC/CCT molecular complex responsible for proper protein folding within cells. The study showed that insufficient levels of healthy CCT3 hinder the protein-folding machinery, leading to misfolded actin proteins, which are critical for cell shape and movement. Co-author Tim Schedl noted, "Our studies revealed that the genetic change reduces the activity of the normal protein, decreasing the capacity of the protein-folding machinery."
To broaden their search for additional cases, the researchers analyzed a global database of individuals with intellectual and developmental disabilities, identifying 22 patients with genetic alterations in several CCT proteins forming the protein-folding machine. This discovery suggests a novel category of rare genetic disorders linked to the protein folding process.
The collaborative effort included contributions from RWTH Aachen University in Germany and Stanford University, which helped illuminate the gene’s impact on brain development and its role in protein folding, respectively. Pak emphasized the importance of using model organisms like C. elegans to provide critical insights into human disease mechanisms. With ongoing NIH funding, the team aims to resolve more cases of unexplained genetic conditions, hoping to bring clarity and potential therapies to families facing similar challenges.
Story Source:
Materials provided by Washington University School of Medicine. The original text of this story is licensed under a Creative Commons License. Note: Content may be edited for style and length.
Journal Reference:
- Florian Kraft, Piere Rodriguez-Aliaga, Weimin Yuan, Lena Franken, Kamil Zajt, Dimah Hasan, Ting-Ting Lee, Elisabetta Flex, Andreas Hentschel, A. Micheil Innes, Bixia Zheng, Dong Sun Julia Suh, Cordula Knopp, Eva Lausberg, Jeremias Krause, Xiaomeng Zhang, Pamela Trapane, Riley Carroll, Martin McClatchey, Andrew E. Fry, Lisa Wang, Sebastian Giesselmann, Hieu Hoang, Dustin Baldridge, Gary A. Silverman, Francesca Clementina Radio, Enrico Bertini, Andrea Ciolfi, Katherine A Blood, Jean-Madeleine de Sainte Agathe, Perrine Charles, Gaber Bergant, Goran Čuturilo, Borut Peterlin, Karin Diderich, Haley Streff, Laurie Robak, Renske Oegema, Ellen van Binsbergen, John Herriges, Carol J. Saunders, Andrea Maier, Stefan Wolking, Yvonne Weber, Hanns Lochmüller, Stefanie Meyer, Alberto Aleman, Kiran Polavarapu, Gael Nicolas, Alice Goldenberg, Lucie Guyant, Kathleen Pope, Katherine N. Hehmeyer, Kristin G. Monaghan, Annegret Quade, Thomas Smol, Roseline Caumes, Sarah Duerinckx, Chantal Depondt, Wim Van Paesschen, Claudine Rieubland, Claudia Poloni, Michel Guipponi, Severine Arcioni, Marije Meuwissen, Anna C. Jansen, Jessica Rosenblum, Tobias B. Haack, Miriam Bertrand, Lea Gerstner, Janine Magg, Olaf Riess, Jörg B. Schulz, Norbert Wagner, Martin Wiesmann, Joachim Weis, Thomas Eggermann, Matthias Begemann, Andreas Roos, Martin Häusler, Tim Schedl, Marco Tartaglia, Juliane Bremer, Stephen C. Pak, Judith Frydman, Miriam Elbracht, Ingo Kurth. Brain malformations and seizures by impaired chaperonin function of TRiC. Science, 2024; 386 (6721): 516 DOI: 10.1126/science.adp8721

0 Comments